How many days do ammonia vapors remain in the air? The influence of pollution sources on the natural environment and the human body
(from the Greek hals ammoniakos - ammonia) - NH3, a colorless gas with a pungent suffocating odor; density 0.681 g/cm³ (-33.35 C), melting point -77.7 C, boiling point -33.35 C; at a pressure of 0.9 MPa it liquefies at room temperature. Very soluble in water; aqueous solution - ammonia. Obtained by catalytic synthesis from nitrogen and hydrogen under pressure. They are used in the production of nitric acid and fertilizers (4/5 of the ammonia produced), ammonium salts, hydrocyanic acid, and soda. Liquid ammonia is a refrigerant and highly concentrated fertilizer. Explosive. Toxic.
Ammonia is a colorless gas with a pungent odor, which is a combination of nitrogen and hydrogen.
Ammonia: why it is dangerous and what to do in case of poisoning.
Ammonia translated from Greek (hals ammoniakos) means ammon's salt. Ammonia is a colorless gas with a pungent odor, melting point - 80 ° C, boiling point - 36 ° C, soluble in water, alcohol and a number of other organic solvents. Synthesized from nitrogen and hydrogen. In nature, it is formed during the decomposition of nitrogen-containing organic compounds.
Pure ammonia was obtained by the English chemist and philosopher Joseph Priestley in 1774. The industrial technology for producing ammonia was developed and implemented in 1913 by German chemists Fritz Haber and Carl Bosch, who received Nobel Prizes for their research.
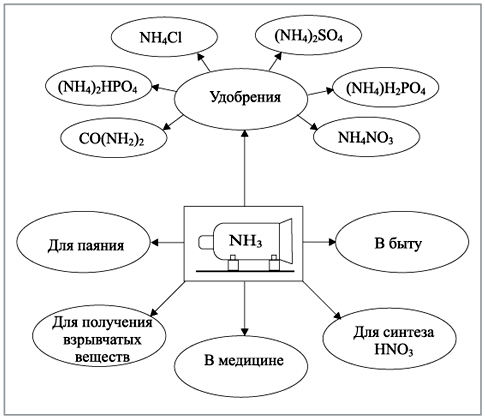
Ammonia is one of the most important products of the chemical industry. Most of The ammonia obtained in industry is used to prepare nitric acid, nitrogen fertilizers, and dyes. Ammonia is also used to produce explosives. Aqueous solutions of ammonia are widely used. As a weak volatile base, it is used in chemical laboratories and industries. Baking soda is produced using ammonia.
In medicine, a 10% aqueous solution of ammonia is known as ammonia. The pungent odor of ammonia irritates specific receptors of the nasal mucosa and promotes stimulation of the respiratory and vasomotor centers, therefore, in case of fainting or alcohol poisoning, the victim is allowed to inhale vapors of ammonia.
When soldering metals, ammonium chloride is used - ammonia - NH4Сl. At high temperatures, ammonia decomposes to form ammonia, which cleans the surfaces of the soldering iron and the product being soldered from metal oxides.
When liquid ammonia evaporates, it is absorbed a large number of heat, which is why it is used in refrigeration units.
Liquid ammonia causes severe skin burns, so it is usually transported in steel cylinders (painted yellow, have the inscription “Ammonia” in black), railway and road tanks, by water - in special tankers, and are also transported through pipelines.
A mixture of ammonia and air is explosive. Ammonia burns in the presence of a constant source of fire. Containers may explode when heated. Ammonia gas is a toxic compound. When its concentration in the air of the working area is about 350 mg/m3 (milligrams per cubic meter) or higher, work should be stopped and people should be removed from the danger zone. The maximum permissible concentration of ammonia in the air of the working area is 20 mg/m3.
Ammonia is dangerous if inhaled. In acute poisoning, ammonia affects the eyes and Airways, at high concentrations it is possible fatal outcome. Causes severe coughing, suffocation, and with a high concentration of vapors - agitation, delirium. Upon contact with skin - burning pain, swelling, burn with blisters. In case of chronic poisoning, indigestion, catarrh of the upper respiratory tract, and hearing loss are observed.
In case of ammonia poisoning, the following measures must be taken.
First aid: rinse eyes and face with water, put on a gas mask or a cotton-gauze bandage moistened with a 5% solution of citric acid, rinse exposed skin with plenty of water, immediately leave the source of infection.
If ammonia gets into the stomach, drink several glasses of warm water with the addition of one teaspoon of table vinegar per glass of water and induce vomiting.
Personal protection: insulating and filtering gas masks of brands M, KD, RPG-67KD respirator, in their absence - a cotton-gauze bandage moistened with a 5% solution of citric acid, a protective suit, rubber boots, gloves.
In the affected area, you must stay to the windward side. Isolate the danger area and keep outsiders away. Enter the accident zone only in full protective clothing. Follow the measures fire safety, no smoking.
In case of leakage or spillage: eliminate sources of open flame. Fix the leak. Use sprayed water to precipitate gases. Notify local authorities of the danger of poisoning. Evacuate people from the area exposed to the danger of poisonous gas. Do not allow the substance to enter water bodies, tunnels, basements, or sewers.
In case of fire: remove from the fire area if it does not pose a danger and allow it to burn out. Do not get close to burning containers. Cool containers with water from a maximum distance. Extinguish with sprayed water and air-mechanical foam from a maximum distance.
Ammonia can easily be called one of the most important products of the chemical industry.
Most of the gas that is produced industrial enterprises, is used for the production of nitric acid and various kinds of dyes. Is ammonia harmful to humans? Rarely does anyone think about this question.
More about ammonia
LOSE TWO SIZES IN A MONTH!
The formula for losing weight is simple - burn more calories than you take into your body. But how can this be achieved in practice? Depleting yourself with complex and often dangerous diets is very risky. Spending a lot of money and time on the gym is not something everyone can afford. Kartunkova named the mistake of all those who LOSE WEIGHT: “Girls, just lose weight, here’s the recipe: before breakfast...”
Ammonia - like most similar compounds - is a transparent gas that is highly soluble in water, alcohol solutions and many organic solvents. In industry, it is extracted by synthesis from two elements - nitrogen and hydrogen, which in nature correspond to simple substances - nitrogen and hydrogen, respectively.
The industrial extraction process is characterized by complex physical and chemical laws that increase the yield of the finished product. In nature, ammonia is a product of the decomposition of nitrogen-containing substances.
In the medical field, ammonia is used in the form of a 10 percent solution, which is popularly called “ammonia.” A sharp “aroma” in people can excite nasal receptors and trigger excitatory processes that are transmitted along nerve fibers to the hindbrain.
It contains structures that are responsible for breathing and heart function - which is why ammonia is used to bring a person to consciousness in case of fainting or intoxication of any kind.
In the soldering process, another derivative of ammonia is used - its salt of hydrochloric acid, ammonium chloride. At high temperatures, it decomposes to form ammonia vapor and hydrochloric acid in the form of gas. Ammonia frees the working surface of the soldering iron from metal oxides and particles of products that can be soldered.
Ammonia evaporation is an endothermic process. Thanks to these properties, it has been used in refrigeration units.
Why is ammonia harmful to health?
The harm of ammonia in liquids has long been known. It can cause burns, so it is transported in steel cylinders with special markings - the cylinders are painted yellow and the inscription “Ammonia” is applied in black paint.
The combination of ammonia with ordinary air is dangerous due to the high probability of explosion. Ammonia can burn over an open fire. Vapors of ammonia gases can cause serious poisoning. In case of poisoning, the eyes and mucous membranes of the respiratory tract are primarily affected.
Poisoning at high concentrations can be fatal. All the harmfulness of the gas lies in the fact that irritation of the mucous membrane of the respiratory tract triggers a severe coughing attack; with increasing concentration, the nervous system is affected - the person begins to delirium, his actions and words become inadequate.
When ammonia comes into contact with the skin, it provokes an attack of severe pain, and a burn is formed at the site of contact, characterized by large swelling. With chronic poisoning in the body, the digestive system, respiratory tract and nerve fibers will suffer.
In addition to the above, ammonia can cause frostbite, paralysis and even loss of vision. Small amounts of gas cause pain in the eyeballs, and large concentrations can cause burns to the corneal tissue and complete loss of vision.
Ammonia is toxic because its ions in the form of ammonium ions cause alkalinization of the blood plasma. In this case, hemoglobin is able to absorb more oxygen, but it is not able to give it to the cells - hypoxia of the body develops. Ammonia is able to bind glutamic acid and form glutamine, which is known for its osmotic activity. Large amounts of glutamine retain water in cells, causing them to swell and develop edema in a certain part of the body.
If the structures of the nervous system are affected, the brain suffers and death is possible.
First aid for acute intoxication
Prepare for winter!
EVERYONE should know about this! UNBELIEVABLE BUT TRUE! Scientists have developed a unique remedy that helps restore immunity and prepare it for various attacks of influenza viruses and even restore it if you are already sick. Autumn, winter and spring are coming - this is the time when flu activity increases, and to protect yourself and your whole family, scientists advise drinking...
You can also be poisoned by ammonia vapor at home, as it is part of various detergents. The 25th ammonia solution is an excellent tool for cleaning equipment from dirt, furniture from “patina of time,” and removing food and paint stains.
It is freely available in stores. It should be stored at home only in tightly sealed containers.
When using popular household cleaning products for tiles and other surfaces, you must wear a mask, as they also contain ammonia.
Inhalation of vapors can cause nausea and dizziness, which can then lead to loss of consciousness.
Actions first before medical care are as follows:
- The contact area must be washed with running or digested water. A person who is in an environment with volatile ammonia needs to arm himself with a gas mask or a gauze bandage that has been treated with a weak solution of citric acid, according to school course chemistry it was studied that ammonia in an aqueous environment will exhibit alkaline characteristics, and will be neutralized by a weak acid with which the dressing is treated.
- If ammonia gets into the gastrointestinal tract, you must take 3-4 glasses of digested water as quickly as possible with the addition of 5-7 milliliters of vinegar per glass of water. The question of whether to induce vomiting is best left to the care of doctors, since the victim will not be able to say exactly how much ammonia entered his body. Induced vomiting can lead to repeated burns of the mucous membrane of the stomach and esophagus, and only aggravate the already difficult condition of the victim.
When in the affected area, you must take the windward side as quickly as possible and follow all safety measures. Under no circumstances should you smoke.
In the event of a leak, every effort must be made to eliminate sources of open flame. In order to precipitate the gas, you can and should spray water. The Ministry of Emergency Situations notifies the authorities and ordinary residents of all incidents, then transports them to safe places. Their further actions are aimed at preventing the entry of harmful substances into open water bodies and sewers near populated areas.
Many people do not like this colorless liquid because of its strong, pungent odor. Some people always have ammonia, mostly for household purposes. Some people prefer combined detergents, where ammonia is present, but its “aroma” is muffled by fragrances.
What is this substance
To put it in a very scientific way, ammonia is ammonium hydroxide or an aqueous solution of ammonia, usually in a 10% concentration. His chemical formula: NH 4 OH. The use of ammonia is quite varied.
In medicine
Ammonia on a ball of cotton wool is the first aid to a person who feels sick. This remedy is very popular among many doctors for its high effectiveness. Ammonia vapor penetrates the nasopharynx, they irritate the nerve endings and the respiratory center. The person takes a quick breath and, as a rule, comes to his senses.
Many people say that the smell of ammonia is the quickest way to “pass out.”  he is very sharp. This is partly true. If you inhale pure ammonia vapor, breathing can stop completely. This is the body's reflex reaction.
he is very sharp. This is partly true. If you inhale pure ammonia vapor, breathing can stop completely. This is the body's reflex reaction.
In surgery, there is a method of sterilizing hands, which involves the use of ammonia. The technique takes into account the antimicrobial properties of this solution. Another external use of ammonium hydroxide mixed with lanolin is against itching from insect bites.
Ammonia is also taken orally during alcohol intoxication ( folk remedy). In this case, you need to take 5-10 drops of the solution in half a glass of water and drink. It is believed that then the symptoms will decrease. There are also medications that contain an aqueous solution of ammonia.
At home
Fifteen or twenty years ago, it was rare that a housewife would not use ammonia to clean windows. The glass simply sparkled, although the washers had to deal with stains. According to the manufacturers, ammonia solution is still included in some detergents.
 Water and ammonia are great for cleaning painted surfaces. Here are some other areas of household use:
Water and ammonia are great for cleaning painted surfaces. Here are some other areas of household use:
- cleaning fur and suede;
- giving freshness to carpets and rugs;
- in combination with glycerin – removes tea stains from clothes and furniture upholstery;
- cleaning the umbrella fabric;
- eliminating shiny, greasy stains (for example, on shirt collars).
What is the danger of ammonia
In addition to the harmful effects of vapors, ammonia is highly irritating to the mucous membranes, as well as the skin, and can cause burns. Therefore, the ammonia aqueous solution should be kept away from children, and it should be used very carefully. It is also important to know what first aid should be if trouble does happen.
Poisoning
If this product is used incorrectly, intoxication may occur. Medical practice records various methods of poisoning.
In production
 Enterprises that use an aqueous solution of ammonia in various concentrations are the first risk group. Depending on the volume of the substance and how it is used, two options are possible. The first is in pairs.
Enterprises that use an aqueous solution of ammonia in various concentrations are the first risk group. Depending on the volume of the substance and how it is used, two options are possible. The first is in pairs.
The second is direct contact of the skin and mucous membranes with the solution.
That is why employees of enterprises, even in fully automated production facilities, always undergo special training. This allows you to prevent many dangerous situations and serious consequences.
At home
Respiratory intoxication at home occurs quite rarely. If you happen to spill ammonia, you can take action by  few seconds. "Breathe in" until negative consequences it is practically impossible. Brief contact between normal skin and ammonia is completely harmless. But if your skin is sensitive and/or has scratches, you will definitely need gloves.
few seconds. "Breathe in" until negative consequences it is practically impossible. Brief contact between normal skin and ammonia is completely harmless. But if your skin is sensitive and/or has scratches, you will definitely need gloves.
And another category of poisoning is the accidental, thoughtless or, unfortunately, intentional ingestion of ammonia. Here the consequences can be very different. Everything will depend on the quantity and general condition of the poisoned person. In any case, every person must know what first aid should be for alcohol.
Symptoms of intoxication

What to do in case of poisoning
The outcome of various emergency situations almost always depends on how quickly and how correctly first aid was provided. In mild cases, all measures can be taken independently. But only when the victim’s condition does not cause concern. Do not hesitate to call an ambulance if you have any suspicions.
In case of inhalation of vapors and minor burns
 In case of respiratory intoxication, first aid is simple: you need to provide access fresh air and eliminate the source of ammonia fumes.
In case of respiratory intoxication, first aid is simple: you need to provide access fresh air and eliminate the source of ammonia fumes.
If the negative impact was short-term, then the consequences will be insignificant. Medical assistance in this case, it is necessary if there is delirium or a sharp deterioration in health.
Rinsing with plenty of water is the first step that is needed if ammonia gets on your skin or eyes. Most often this is the limit. However, if vision deterioration occurs or the skin at the burn site is irritated for a long time, a doctor will be needed.
When taken orally
In this case, the main thing is to call the ambulance. First aid depends on some symptoms. If unbearable pain is felt behind the sternum and/or in the stomach, this may indicate perforation. In this case, you should not drink.
If there is no suspicion of perforation, then the main measures are as follows:
- gastric lavage with warm water or a solution of citric or acetic acid (1%);
- ingestion of lemon juice or an acidic solution (citric, acetic acid, concentration 2-3%) one tbsp. l. every five minutes;
- Under no circumstances should you rinse your mouth with a soda solution.
Treatment
Medical care consists of eliminating the consequences of poisoning and normalizing the activity of the affected organs.
Poisoning with ammonia has serious consequences for the victim’s body. Ammonia is found in every home. Some people use it at home to wash windows, as a stain remover for carpets and fabrics, and others use it as a first aid product. Ammonia should be kept away from children and used with caution in everyday life. It is not uncommon for small children to open a poorly closed bottle of ammonia and inhale ammonia fumes or spill it on the floor or furniture. Every parent should know what to do in this case and how to prevent ammonia poisoning.
Ammonia has found its use as a means of quickly bringing a person to consciousness in case of fainting or other causes of loss of consciousness. However, many say that The smell of ammonia will make you faint faster than you will wake up from it. This judgment can also be considered correct, since when inhaling pure ammonia vapor a person may stop breathing.
Poisoning with ammonia
 Ammonia emits harmful fumes that not only irritate mucous membranes and skin, but can also cause burns. Various cases of ammonia intoxication are known in medicine. Those who work with it on an industrial scale are most susceptible to ammonia poisoning. There are 2 possible options for ammonia poisoning at work:
Ammonia emits harmful fumes that not only irritate mucous membranes and skin, but can also cause burns. Various cases of ammonia intoxication are known in medicine. Those who work with it on an industrial scale are most susceptible to ammonia poisoning. There are 2 possible options for ammonia poisoning at work:
- ammonia vapor poisoning;
- burns of the skin and mucous membranes with ammonia solution.
Therefore, at those enterprises whose activities involve ammonia, workers undergo mandatory training on working with toxic substances.
In everyday life, ammonia poisoning occurs very rarely. Even if you spill a jar of ammonia solution, such a quantity of toxic vapors will not be enough to cause intoxication. In addition, if you quickly open the windows and wipe up spills, even mild vapor poisoning can be avoided. Brief contact of ammonia with skin is also not dangerous. If there are scratches and wounds on the skin, then it is best to wear gloves when coming into contact with ammonia.
But sometimes a person commits a rash, deliberate act - taking ammonia inside. In this case, the consequences can be very serious.
Symptoms of ammonia poisoning
Intoxication with ammonia occurs if:
- inhale an aqueous solution of ammonia for a long time;
- ammonia contacts the skin for a long time;
- ammonia splashed into the eye;
- household ammonia was drunk knowingly or thoughtlessly;
- undiluted ammonia was drunk.
 There will be no harm to the body if you inhale ammonia vapor for a short time - literally a few seconds. Then, with each breath, the concentration of ammonia in the lungs increases, causing irritation of the respiratory tract. One of the first signs of ammonia vapor poisoning is suffocation, accompanied by a continuous cough. Ammonia fumes are irritating nervous system, therefore, when intoxicated by vapors, a person may be overly excited. Ammonia vapors entering the lungs lead to local allergic-type edema. This is a very serious situation, as it leads to bronchospasm (compression of small-caliber bronchi) and pulmonary ischemia (vessels are greatly constricted).
There will be no harm to the body if you inhale ammonia vapor for a short time - literally a few seconds. Then, with each breath, the concentration of ammonia in the lungs increases, causing irritation of the respiratory tract. One of the first signs of ammonia vapor poisoning is suffocation, accompanied by a continuous cough. Ammonia fumes are irritating nervous system, therefore, when intoxicated by vapors, a person may be overly excited. Ammonia vapors entering the lungs lead to local allergic-type edema. This is a very serious situation, as it leads to bronchospasm (compression of small-caliber bronchi) and pulmonary ischemia (vessels are greatly constricted).
It is important to note that this is only possible with prolonged inhalation of ammonia (more than 3-5 seconds). It turns out that the longer ammonia vapor enters the respiratory system, the more serious the prognosis for the patient becomes. After just 40 seconds of inhalation, the first symptoms of damage to the central nervous system appear.
When ammonia gets on the skin and with prolonged contact with it, swelling forms at the site of contact. At this time the person feels severe itching and burning..
It happens that when you open a jar of ammonia, a splash of the solution gets into your eyes. In this case, a corneal burn occurs. In this case, the person feels severe pain and discomfort, which is accompanied by lacrimation.
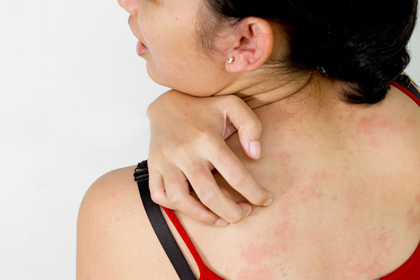 In this case, the severity of poisoning will depend on the concentration and amount of ammonia solution drunk. Poisoning occurs if a person has individual intolerance, or the concentration of ammonia in the solution is exceeded. The minimum that a poisoned person can get by with is vomiting with the smell of ammonia, sharp pain in the stomach, severe diarrhea, thirst, swelling of the larynx, inflammation of the nasopharynx and respiratory tract. The maximum that can happen is inevitable damage to the liver, kidneys and lungs.
In this case, the severity of poisoning will depend on the concentration and amount of ammonia solution drunk. Poisoning occurs if a person has individual intolerance, or the concentration of ammonia in the solution is exceeded. The minimum that a poisoned person can get by with is vomiting with the smell of ammonia, sharp pain in the stomach, severe diarrhea, thirst, swelling of the larynx, inflammation of the nasopharynx and respiratory tract. The maximum that can happen is inevitable damage to the liver, kidneys and lungs.
If ammonia was drunk undiluted, then burns of the pharynx, esophagus, larynx, and lips are added to the above symptoms. Swelling can be such that it blocks the larynx and trachea. At this time, a person experiences terrible pain, sometimes leading to painful shock. About 5% of cases are fatal. The minimum dose at which a person will die is 50 ml.
What to do if you are poisoned with ammonia
The outcome of each situation depends on how quickly and correctly first aid is provided. In mild cases, you can do without calling an ambulance. But provided that the patient’s condition is not critical or severe. In any case, after even mild poisoning, you should consult a doctor.
When intoxicated by ammonia vapor, the first step is to provide the victim with access to fresh air - open the vents or windows, or, in extreme cases, take the person outside.
If ammonia gets on your skin or eyes, wash the burn area with plenty of cold water. If, when ammonia solution gets into the eyes, the victim’s vision sharply deteriorates, it is necessary to immediately consult an ophthalmologist.
 When ingesting an ammonia solution, you must first call an ambulance. It is necessary to ask the victim about the symptoms: if there is a feeling of severe unbearable pain in the stomach or behind the sternum, this indicates perforation. In this case, you should absolutely not drink. If there is no perforation, then it is necessary to rinse the stomach with warm water or a solution of citric acid before the ambulance arrives. Next, until the ambulance arrives, you need to drink 1 tbsp every 5 minutes. l. lemon juice or low concentration citric acid. Under no circumstances should you rinse a patient’s mouth with a soda solution.
When ingesting an ammonia solution, you must first call an ambulance. It is necessary to ask the victim about the symptoms: if there is a feeling of severe unbearable pain in the stomach or behind the sternum, this indicates perforation. In this case, you should absolutely not drink. If there is no perforation, then it is necessary to rinse the stomach with warm water or a solution of citric acid before the ambulance arrives. Next, until the ambulance arrives, you need to drink 1 tbsp every 5 minutes. l. lemon juice or low concentration citric acid. Under no circumstances should you rinse a patient’s mouth with a soda solution.
If vapor poisoning occurs at work, the following rules must be followed:
- remove the victim from the source of poisoning by putting on a respirator;
- rinse the damaged areas of the skin with plenty of water, and rinse the eyes too;
- rinse the stomach, mouth, nasopharynx (before rinsing the stomach, exclude perforation);
- inhale oxygen or vapors of acidic solutions to neutralize ammonia vapors;
- Give lemon juice or acidic solution in small portions.
Treatment after poisoning with ammonia
Treatment of the consequences of ammonia poisoning consists in eliminating toxins and normalizing functions internal organs, affected by ammonia. For treatment, painkillers, means for treating burns, and relieving swelling are used. If ammonia enters the body, a strict diet is prescribed to normalize the functions of the organs that are most affected by the toxic effects of ammonia: the kidneys, liver and stomach. Treatment is prescribed that removes toxic substances from the intestines. As a rule, poisoning in this case is accompanied by severe diarrhea, possibly with blood. If the poisoning affects the esophagus, then there is a high probability of cicatricial stenosis. The consequences of severe ammonia poisoning, for example at work, can include tremors, loss of the senses of touch, hearing, vision and memory loss.
For those people who see their surroundings as black and white, there are clear distinctions between useful and harmful to humans things. In fact, our world is more complex, multicolored and multifaceted. Something useful under certain conditions and in quantities exceeding permissible doses turns out to be deadly, and snake venom, for example, is used for medicinal purposes and serves to benefit people. The same can be said about ammonia.
There is a version that ammonia vapor in the form of ammonia was used by ancient Egyptian shamans, thus creating for themselves the conditions of an altered state of consciousness when carrying out ritual rites. Worshiping their god Amon, they named the gas “ammoniac” in his honor.
According to another version, ammonia was discovered in the Ammon oasis, located in the north of the American continent, at the largest crossroads of caravan roads. People and animals stopped there to rest. From rotting urea and other waste products of their organisms, in hot climates, a gas with a pungent specific odor soon began to be released, named ammonia in honor of the oasis.
In the distant Middle Ages, alchemists were able to isolate “alkaline air,” as they called it, by combining hydrogen and nitrogen. Reserves of Chilean saltpeter at that time were already depleted, so the issue of chemical synthesis of ammonia for industrial needs became increasingly urgent. And only at the beginning of the last century F. Haber received Nobel Prize for the creation of a small contact apparatus in which, thanks to increased pressure, the process of ammonia synthesis was carried out.
General characteristics of gas

The slightly alkaline properties of an aqueous solution of ammonia are used to produce ammonia, which is widely used in medicine, as well as to clean oxides from metal surfaces that need to be soldered. When interacting with acids with the help of ammonia, ammonium salts are obtained, and with metals - amides, aqueous solutions of which are excellent conductors of electric current.
When ammonia is oxidized with air on platinum catalysts, nitrogen oxides are obtained, which are necessary in the chemical industry to create agricultural fertilizers. As ammonia heats up in an oxygen atmosphere, it burns, breaking down into nitrogen and water. In liquid form, ammonia is used as a solvent and refrigerant in refrigeration units, and is also used to produce explosives and soda, nitric acid and polymers.
Ammonia is a colorless gas with a characteristic and pungent odor. Ammonia has the smell of ammonia. Ammonia is the final product of nitrogen metabolism in a living organism. Ammonia is formed as a result of the transformation of amino acids, proteins and other compounds. Ammonia is very toxic to the body; part of it is converted by the liver into a harmless, less toxic compound - urea (urea). Urea is excreted by the kidneys.

The properties of ammonia in the body are also associated with the resynthesis of amino acids from ammonia and amino acid analogues. The properties of ammonia when exposed to the body have a suffocating and neurotropic effect; when exposed to inhalation, ammonia can cause toxic lung damage, as well as severe damage to the nervous system.
Ammonia vapors have an irritating effect on the mucous membranes of the eyes, skin, and respiratory organs. Ammonia has a rather pungent odor that humans can smell. Ammonia vapor can cause severe lacrimation, pain in the eyes, seizures, burns of the conjunctiva and cornea, decreased vision, redness of the skin and itching.
Contact of liquefied ammonia with the skin causes a burning sensation, a chemical burn of the skin with blisters and even ulcerations. Liquefied ammonia absorbs heat during evaporation, so contact with skin can cause frostbite.
In medicine, ammonia is used to stimulate breathing in fainting situations and to stimulate vomiting. It is used externally for insect bites, myalgia, and neuralgia. The surgeon's hands are treated with ammonia. A high concentration of ammonia causes a reflex cessation of breathing; if it enters the esophagus and stomach, it provokes, and damage to the mucous membrane of the eyes can lead to complete loss.
It is necessary to rinse thoroughly and widely open eyes running water, and if ammonia gets on the skin, apply lotions made from an aqueous solution of citric or acetic acid to the affected areas. If ammonia vapor gets into the respiratory tract, you need to provide access to fresh air and give the victim warm milk and soda. Spasms during suffocation are relieved by warmth in the neck and sedatives.
By taking precautions when working with ammonia, you can protect yourself from it. negative impact using beneficial features of this substance.
For example, fresh flowers lightly treated with ammonia solution begin to smell fragrant, even if in nature they did not have a pronounced aroma. Florists also use the ability of ammonia to change the color of petals to create fantastic compositions. Truly, the beautiful and the ugly are always nearby, and the benefits and harms are relative.
♦ Category: .
Read for Health one hundred percent:

- Puff pastries stuffed with stewed cabbage
- Recipe: Sponge cake "Apple" - "in the oven"
- Chicken hearts in sour cream sauce
- How to cook bacon and eggs
- How to cook minced meat with vegetables in a hurry
- Gemini - their compatibility with other signs in love
- Submitting an application for the Unified State Exam: deadlines and features of the procedure
- Meaning of the female name hope
- Russian schoolchildren were reminded of what they can and cannot bring with them to the OGE
- Leo in the year of the Rooster: characteristics of men and women in love and business relationships
- Why do you dream of a blooming apple tree: interpretation options according to dream books Seeing a blooming apple tree in a dream
- Crab salad with cheese - five best recipes
- Cutlets in foil in the oven
- Salad of crab sticks with corn, cheese and egg Crab salad with hard cheese
- Potatoes with minced meat in the oven in foil
- Cutlets in foil in the oven
- Minced meat in foil in the oven with filling
- Pearl barley porridge with beef
- Recipes for baked apples with cottage cheese, raisins, honey, nuts and cinnamon
- You can get better from potatoes









