Characteristics of artificial fabrics. “Types of chemical fibers. Properties of chemical fibers and fabrics from them
7th grade
Topic: "Properties of chemical fibers and fabrics from them."
Goals and objectives:
educational
To give an idea about the types of chemical fibers, to acquaint with the methods of their production, properties and processing technology and application in the surrounding life.
Educational
Learn to understand the properties of tissues and apply this knowledge in life.
Develop the ability to analyze and compare, observation and attention.
Educational
Education of activity, accuracy, ability to work in a group.
Equipment :
Fabric Collection, Handout, Cards, Safety Instructions, Classification Chart textile fibers”, computers, multimedia installation, computer presentation
Lesson type: lesson of studying and primary consolidation of new knowledge
Methods: problem-search, information-developing, reproductive, creative-reproductive.
Work in teams (3 teams - according to the number of rows in the office).
During the classes.
I. Organizational moment.
Check readiness for the lesson.
Preparing students for learning.
2 . Knowledge update according to the previous learning material. (Work in teams).For each correct answer, the team gets a bonus / at the end of the lesson - grades /.
Questions:
Blitz Poll:
(Slide 2.3)
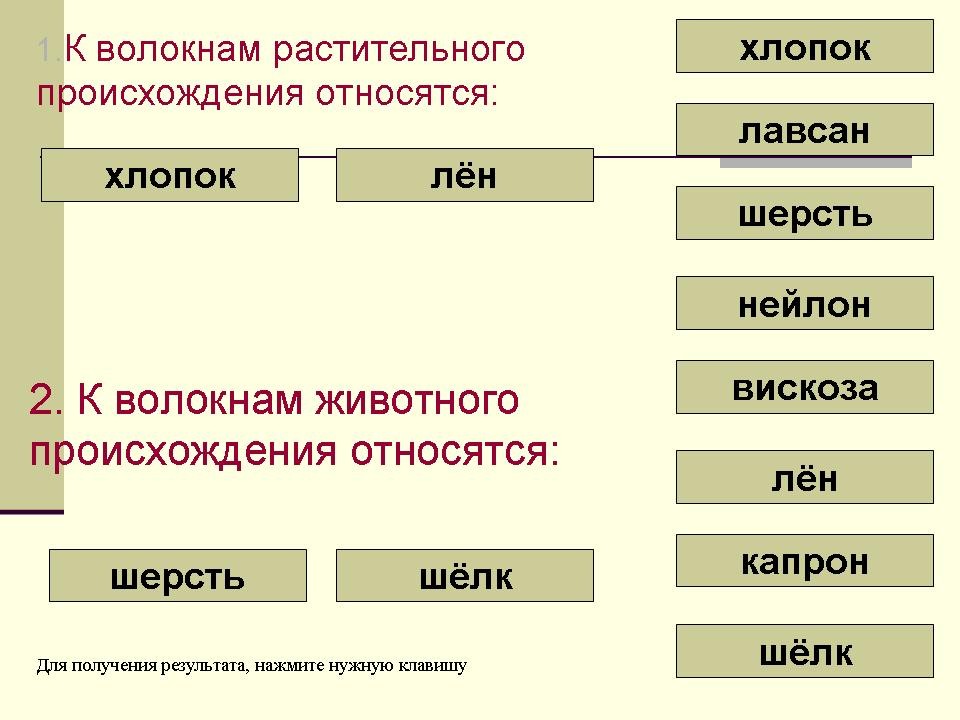
1. Complete the sentences:
1. Cotton and linen are fibers (of plant origin)
2. Animal fibers include (wool and silk)
2. Make a daisy chain fabric making:
Plant - fiber - yarn - fabric
3. Fill in the missing words.
The thinnest fiber (silk)
Smoothest fiber (linen)
The shortest fiber (cotton)
The fluffiest fiber (wool)
4. Significant hygroscopicity (all natural fiber fabrics)
5. Have a large dust capacity (woolen fabrics)
6. They drape better than others (silk fabrics)
3. Learning new material.
Motivation learning activities students
Introductory speech of the teacher:
- Have you ever wondered whypeople began to look for raw materials from which it would be possible to obtain fabric in a cheap way, warm like wool, light and beautiful like silk, practical like cotton?
Today I will tell you and at the end of the lesson you will answer problematic issue:
1. Verbal and illustrative story (Slide 4).
Teacher. Since ancient times, for the production of fabrics, people used those fibers that nature gave them. At first, these were fibers of wild plants, then fibers of hemp, flax, and also animal hair. With the development of agriculture, people began to grow cotton, which gives a very durable fiber.
But natural raw materials have their drawbacks, natural fibers are too short and require complex technological processing. And, people began to look for raw materials from which it would be possible to obtain fabric in a cheap way, warm like wool, light and beautiful like silk, practical like cotton.
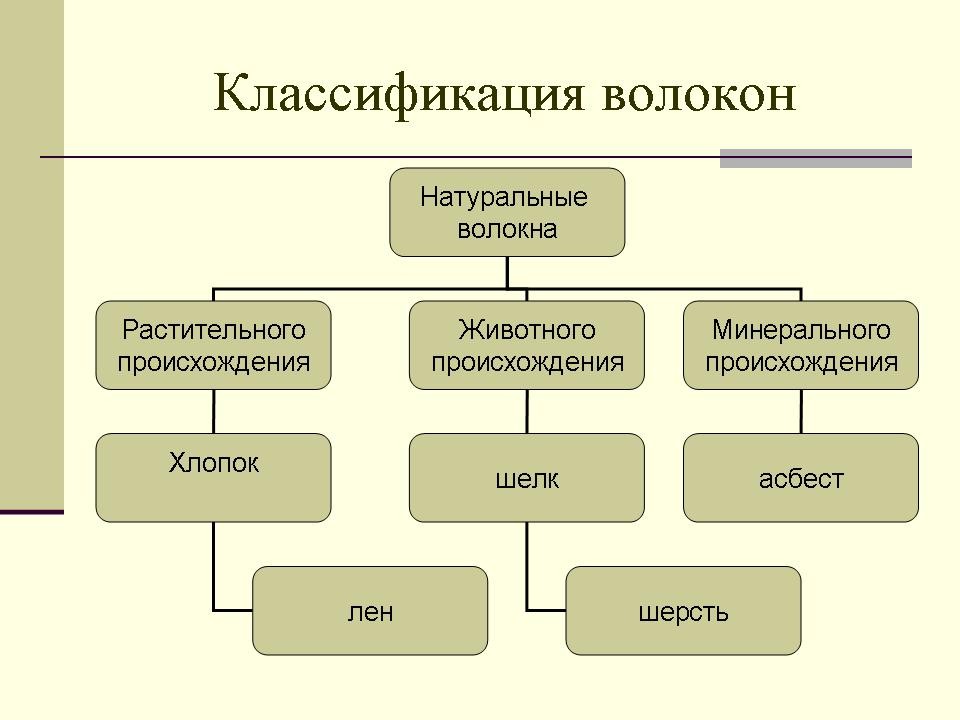
Today, all textile fibers can be represented in the form of the following diagram (Slide 5).
Now more and more new types of chemical fibers are being synthesized in laboratories, and not a single specialist can enumerate their vast multitude. Scientists managed to replace even wool fiber - it is callednitron .
The production of chemical fibers includes 5 stages: (Slide 6.7)
1. Receiving and pre-treatment of raw materials.
2. Preparation of spinning solution or melt.
3. Thread forming.
4. Finishing.
5. Textile processing. Cotton and bast fibers contain cellulose. Several methods were developed to obtain a solution of cellulose, forcing it through a narrow hole (die) and removing the solvent, after which threads similar to silk were obtained. Acetic acid, alkaline copper hydroxide solution, sodium hydroxide, and carbon disulfide were used as solvents. The resulting threads are called, respectively: acetate, copper-ammonia, viscose.
When molded from solutionwet In this way, the jets enter the solution of the precipitation bath, where the polymer is released in the idea of the thinnest threads.
big group The filaments coming out of the spinnerets are drawn, twisted together and wound as a complex filament onto a cartridge. The number of holes in the spinneret in the production of complex textile yarns can be from 12 to 100.
In the production of staple fibers, the spinneret can have up to 15,000 holes. A flagellum of fibers is obtained from each spinneret. The tows are connected into a tape, which, after pressing and drying, is cut into bundles of fibers of any given length. Staple fibers are processed into yarn in pure form or mixed with natural fibres.
Synthetic fibers are made from polymeric materials. Fiber-forming polymers are synthesized from oil refining products: benzene, phenol. ammonia, etc.
Presentation of groups with pre-prepared information:
1 group:
By changing the composition of the feedstock and the methods of its processing, synthetic fibers can be given unique properties that natural fibers do not have. Synthetic fibers are obtained mainly from the melt, for example, fibers from polyester, polyamide, pressed through spinnerets.
Depending on the type of chemical raw material and the conditions of its formation, it is possible to produce fibers with a variety of predetermined properties. For example, the stronger you pull the jet at the moment it exits the spinneret, the stronger the fiber is. Sometimes chemical fibers even outperform steel wire of the same thickness.
2 group:
Among the new fibers that have already appeared, one can note fibers - chameleons, the properties of which change in accordance with changes in environment. Hollow fibers have been developed into which a liquid containing colored magnets is poured. Using a magnetic pointer, you can change the pattern of a fabric made from such fibers.
Since 1972, the production of aramid fibers has been launched, which are divided into two groups. Aramid fibers of one group (nomex, conex, phenylone) are used where resistance to flame and thermal effects is required. The second group (Kevlar, Terlon) has high mechanical strength combined with low weight.
3rd group:
High mechanical strength and good resistance to chemicals are ceramic fibers, the main form of which consists of a mixture of silicon oxide and aluminum oxide. Ceramic fibers can be used at temperatures around 1250oC. They are distinguished by high chemical resistance, and radiation resistance allows them to be used in astronautics.
Familiarization with various properties textile fibers
(Slide 8*)
Table "Classification of fabrics by fibrous composition" (It can be printed out by the number of students and handed out to strengthen it in a notebook, in order to save time).
| Fabric name | Positive properties | Negative properties |
| They have good strength, lightness, softness. They easily absorb moisture, allow air to pass through, are easy to wash and do not crumble when cutting. Easy to smooth out. | They crumple a lot |
|
| linen fabrics | They have high strength. They pass air well, absorb moisture and do not crumble. Easy to smooth out. | They are hard, thick, strongly crumpled. |
| Woolen fabrics | Very warm, drape well, little wrinkle. | When soaking, they change their size, i.e. "sit down" |
| Silk fabrics | Durable, they absorb moisture well, dry quickly, freely pass air, and wrinkle little. | They stretch and crumble when cut. |
| artificial fabrics | Sturdy, they drape well. They are hygroscopic. | They wrinkle strongly. When wet, they lose their strength. When cutting - crumble. |
| Synthetic fabrics | They have elasticity and strength. Do not wrinkle, do not shrink, retain their shape well. | They do not absorb moisture well and crumble heavily when cutting. |
4.Laboratory - practical work.
“Determination of the raw material composition of materials and the study of their properties” (Work in teams). (Slide 9)
In the lesson, during laboratory work, you will see in practice what properties fabrics made from chemical fibers have and how to properly care for products made from such fabrics.
Tools and materials: samples of fabrics from artificial and synthetic fibers, wool, cotton; needle; a vessel with water; crucibles for ignition of threads.
(Slide 10).
"Table of properties of chemical fibers"
| Fiber | Shine | crimp | Strength | Wrinkle | Combustion |
| viscose | cutting | No | big | burns well, gray ash, burnt paper smell. |
|
| acetate | matte | No | decreases when wet | less than viscose | quickly burns with a yellow flame, a melted ball remains |
| capron | cutting | No | high | very small | melts to form a solid ball |
| lavsan | weak | eat | high | very small | burns slowly, forms a solid dark ball |
| nitron | weak | eat | high | very small | burns with flashes, a dark influx is formed |
Progress (Slide 11).
Consider appearance tissue samples. Determine which of them have a shiny surface, and which have a matte surface.
Determine by touch the degree of smoothness and softness of each sample.
Determine the wrinkle of the samples: hold the sample in your fist for 30 seconds, and then open your palm.
Remove 2 threads from each sample and wet one of them. Break the dry thread and then the wet thread. Determine how the strength of the thread changes in this case.
Remove one more thread from each sample and light it in the crucible. Analyze the type of flame, smell and remaining ash after burning.
Record the results of the experiments in the table.
Based on the data obtained and the table of properties of chemical fibers, determine raw material composition each sample.
| tissue sign | Sample #1 | Sample #2 | Sample #3 | Sample #4 |
| Shine | ||||
| Smoothness | ||||
| Softness | ||||
| Wrinkle | ||||
| shattering | ||||
| Wet strength | ||||
| Combustion | ||||
| Raw material composition |
5. Consolidation of the studied material.
1. Control of students' knowledge. (Slide 12).
In order to consolidate new knowledge, the girls respond totest
1. High shedding of threads in fabrics:
A) cotton
B) woolen
B) synthetic
2. Heat-shielding properties are higher at:
A) flax
B) silk
B) nitron
3. What fabrics are highly hygroscopic and breathable?
A) natural
B) artificial
4. What fabrics lose strength when wet?
A) natural
B) synthetic
Evaluation, their reasoning.
2.Competition between teams.
The teams were given envelopes with tissue samples, it is necessary
sort them into two groups:
1.made from natural fibers;
2. made of chemical fibers.
V. Summing up.
Output: the ability to determine the nature of the raw material of the fabric is necessary for subsequent work with the fabric at all stages of the manufacture of the product.
So, our lesson has come to an end, let's remember what we learned in the lesson? Who will answer the problem?What fabrics are used in great demand and why? The responses of the teams are discussed and analyzed.
The teacher sums up the lesson, the bonuses earned in the lesson are calculated, marks are given.
The teacher congratulates the team with the most points.6 .Homework.
Make a collection of fabrics.
For creative team: make a crossword puzzle.
7 .Cleaning jobs .


















 Back forward
Back forward
Attention! The slide preview is for informational purposes only and may not represent the full extent of the presentation. If you are interested this work please download the full version.
The purpose of the lesson: to study the properties of fabrics made of chemical fibers and how they are processed.
- educational
To give an idea about the types of chemical fibers, to acquaint with the methods of their production, properties and processing technology and application in the surrounding life.
- Educational
Learn to understand the properties of tissues and apply this knowledge in life.
Develop the ability to analyze and compare, observation and attention.
- Educational
Education of activity, accuracy, ability to work in a group.
Equipment:
Fabric collection, handout, cards, safety instructions, Textile Fiber Classification chart, computers, multimedia installation, computer presentation (Attachment 1), individual tasks on the computer (crossword application 4, test appendix 3), creative work of students (draft booklet application 2)
Lesson type: lesson of studying and primary consolidation of new knowledge
Methods: problem-search, information-developing, reproductive, creative-reproductive.
Lesson #1
I. Organizational moment.
II. Actualization of previously studied knowledge.
III. Learning new material.
IV. Consolidation of the studied material.
V. Summing up.
During the classes
I. Organizational moment.
- Check readiness for the lesson.
- Preparing students for learning. (Slide 1,2,3)
Introductory conversation.
Do you like to dress beautifully? (Slide 4)
How do you think, where does the creation of clothes begin?
What do you usually look for when buying fabric?
Student responses:
I liked some model in a magazine (or on someone) and you need to buy a fabric that is suitable for this model.
You need a thing for a specific purpose, such as a winter skirt, and for this you need to choose a fabric with the appropriate qualities.
II. Actualization of previously studied knowledge.
(Using role playing, poll blitz)
We have two correspondents of our school newspaper “The World of Fabrics” present at the lesson.
They prepared a report "Stories from Grandma's Chest" and designed it as a booklet using the Publisher program. Let's watch and listen to them.
To learn to understand fabrics, you need to know their properties, then you will learn how to properly care for your things and will always be the most fashionable, beautiful and practical.
In the 5th and 6th grades, you got acquainted with tissues of plant and animal origin.
Let's remember what these fabrics are? (Slide 5.6)
Blitz Poll:
(Slide 7.8)
1. Complete the sentences:
1. Cotton and linen are fibers (of plant origin)
2. Animal fibers include (wool and silk)
2. Make a daisy chain fabric making:
Plant - fiber - yarn - fabric
3. Fill in the missing words.
The thinnest fiber (silk)
Smoothest fiber (linen)
The shortest fiber (cotton)
The fluffiest fiber (wool)
4. They have significant hygroscopicity (all fabrics made from natural fibers)
5. Have a large dust capacity (woolen fabrics)
6. Drape better than others (silk fabrics)
With the help of an interactive test, we draw a line on consolidating the previously studied material (Slide 9). In conclusion, I show the table “Classification of natural fibers” (Slide 10)
III. Learning new material.
natural fibers are natural fibers ready to use. They are environmentally friendly and have a beneficial effect on human health, but their production
is a labor intensive and costly process.
And now we are moving on to a new topic.
Write down the topic of today's lesson:
“Properties of fabrics made of chemical fibers”
Chemical fibers are not found in nature, they are produced using special chemical processes in factories (in the form of continuous filaments and staple fibers). Man-made fiber fabrics are produced less labor intensively and more cheaply.
The economic benefit of using chemical fibers is their lower cost, which is explained by significantly lower labor costs for their production. For example, to obtain the same amount of cotton and linen, it is necessary to expend 10 times more labor than to obtain the same amount of viscose staple fiber, and almost 50 times more than to obtain natural silk.
When did fabrics from chemical fibers appear?
Historical background (Slide 11 ):
- It turns out that back in the 12th century, the Englishman Robert Hooke suggested the possibility of obtaining artificial fiber.
- It was obtained industrially only at the end of the 19th century.
- In Russia, the first factory for the production of artificial silk was built in Mytishchi, and in 1913 it gave the first production.
Chemical fibers are divided into artificial and synthetic. Look at the table:

Picture 1
Characteristics of fabrics made of chemical fibers.
Fabrics made of chemical fibers always have a beautiful appearance and high strength, they are resistant to light and are not affected by moths and microorganisms, and they also retain heat very well.
What is the raw material for the production of synthetic and artificial fabrics?
Raw material for production viscose fiber is coniferous wood
trees - spruce chips, cotton waste, from which, after processing, cellulose is obtained in the form of sheets of cardboard. Dissolved cellulose is a viscous liquid - viscose; by pushing it through the spinneret, thin continuous threads of viscose silk are obtained. Viscose fibers are produced not only in the form of continuous threads, but also in short lengths, i.e. staple fibers suitable for the manufacture of both a homogeneous viscose yarn and mixed, with the addition of different fibers to give a variety of properties to fabrics.
I distribute fabric samples and explain the properties of viscose fabrics:
Positive properties: fabrics made of viscose fibers have a beautiful appearance, high strength, they have a rough surface to the touch.
The negative properties of these fabrics is the loss of strength when wet.
How to obtain acetate fibers is the same as the method of obtaining viscose fiber. The only difference is that the pulp produced from wood and cotton waste is treated with acetic essence or sulfuric acid. Vinegar in Latin is “acetum”, from this word the name of the fiber came from - acetate.
The scheme for obtaining fabric from chemical fibers (Slide 13).
We are looking at samples.
I talk about the properties of acetate fabrics:
Fabrics made from acetate fibers are beautiful, have a slightly shiny surface, resemble silk in appearance and feel, are light, soft, drape well, retain their shape, and are not wrinkled.
- The disadvantage of acetate fabrics is the loss of strength when wet, they are poorly breathable and absorb moisture, and are difficult to iron.
Now let's see: "Where are artificial fabrics used?" (Slide 14)
(Blouses, textiles, skirts, trousers)
For the production of synthetic fibers, simple substances (monomers) are used as raw materials, which are a product of processing coal, oil and natural gas (phenol, ethylene, acetylene, methane, etc.) Synthetic fibers are obtained by combining (synthesis) monomers with the formation of a complex substance polymer (“poly” - a lot), so these substances are called synthetic. This is their difference from artificial fibers, for which complex substances (polymers) are used, which are in nature in finished form (wood, cotton fluff).
TO synthetic fabrics relate:
Kapron is the most durable fiber for tearing and abrasion.
The disadvantages of kapron fabrics include: slipping, shedding, thread expansion, so fabrics made from nylon threads are difficult to process.
Lavsan is a very strong and elastic fiber. It is mixed with various fibers to increase the strength and elasticity of fabrics. In its pure form, lavsan is used for the manufacture of threads, lace, technical fabrics, faux fur pile, and carpets. Fabrics with lavsan are afraid of strong moisture and heat.
Nitron is the most resistant and “warmth” fiber, fluffy, matte, looks like wool. Nitron fibers are used in the manufacture knitwear and faux fur.
Woolen fabrics with nitron fibers are strong, wrinkle slightly, but the disadvantage is strong shrinkage during wetting and flaking.
Synthetic fibers have a number of properties that natural fibers do not have: high mechanical strength, elasticity, resistance to chemicals, low creasing, poor flowability, poor shrinkage. All these properties are positive, so synthetic fibers are added to natural ones in order to obtain fabrics with improved quality.
– Negative properties of synthetic fibers are reduced hygroscopicity, low air permeability, high electrification when worn, therefore it is not recommended to wear clothes made from these fabrics for children and people with hypersensitivity to synthetic fibers.
Let's find out "Where are synthetic fabrics used?" (Slide 15)
Working with the textbook:
I suggest that the girls write out from the textbook the positive and negative properties of fabrics made from chemical fibers.
Student work:
Artificial fabrics: viscose, acetate silk
Synthetic fabrics: kapron, lavsan, nitron
Outcome independent work check by (slide 16)
IV. Consolidation of the studied material.
Control of students' knowledge.
In order to consolidate new knowledge, the girls answer the questions of an interactive crossword puzzle. Appendix 4 and test Annex 3.
Crossword Answers:
Vertical: 1. Viscose, 2. Synthetic 3. Wool, 4. Cotton, 5. Linen, 6. Silk, 7. Acetate.
v. Summarizing.
So, our lesson has come to an end, let's remember what we learned in the lesson
and let's summarize.
Conclusion: the ability to determine the nature of the raw material of the fabric is necessary for subsequent work with the fabric at all stages of the manufacture of the product.
And in the next lesson, during laboratory work, you will see in practice what properties fabrics made from chemical fibers have and how to properly care for products made from such fabrics.
Lesson #2
I. Organizational moment.
II. Laboratory work “Determination of the raw material composition and study of their properties”.
III. Consolidation of new material.
IV. Final part.
During the classes
I. Organizational moment
We have studied the properties of fabrics made of chemical fibers, and now in practice we will try to find out: how can these properties be determined, since the ability to determine
the nature of the raw material of the fabric is necessary for subsequent work with the fabric at all stages of the manufacture of the product. When choosing a style of clothing, it is necessary to determine its purpose, and depending on this, choose a suitable fabric that meets certain requirements by property.
The attendants distribute everything necessary for laboratory work (tissue samples, a needle, scissors, a saucer with water, crucibles for lighting threads ..
Induction training.
I repeat the safety rules with the students.
I suggest that the girls open the textbook and familiarize themselves with the task of laboratory work.
P. Laboratory work “Determination of the raw material composition and study of their properties”
During the laboratory work, the students must determine the nature of the raw material and sort the fabric into groups. To determine the raw materials, the students use the organoleptic method of fiber recognition. During work I spend current briefing and monitor compliance with safety regulations.
Having completed laboratory work, the following conclusion can be drawn:
The ability to determine the nature of the raw material of the fabric is necessary for subsequent work with the fabric at all stages of manufacture. When choosing a style of clothing, it is necessary to determine its purpose, and depending on this, choose a suitable fabric that meets certain requirements according to its properties.
III. Consolidation of the studied material.
A group of students was given a task in advance - to conduct a survey among classmates: “Clothes, from what fabrics do my classmates prefer?”. The results of the student's survey were drawn up in a diagram, which we placed in the presentation ( Slide 17).
And now let's listen to practical advice prepared by our masters of the "Home Academy"
How to determine: what fiber is the fabric made of? (Slide 18)
So, you bought a cool blouse and you need to immediately determine what fiber the fabric is made of.
Pull out one thread from the spare patch, which is attached in the seam, and try to set fire to it with a match.
Vegetable fabric (cotton, linen or viscose) will burn quickly, evenly, brightly, the ash will easily crumble, and the smell of burnt paper will remain in the room.
Animal fabric (wool, silk) will burn badly, spreading the smell of burnt bone; a sintered ball will remain at the end of the thread, which, if touched a little, will collapse.
When burning, a thread of acetate silk smells of acetic acid; a dark and hard ball forms at the end of the thread.
When doing these simple experiments, keep in mind that fabrics are often made from mixed fibers.
How to care for fabrics? (Slide 19)
The method of caring for clothes depends on the raw material composition of the fabric from which it is made. Fabrics made from chemical fibers lose their strength when washed, therefore, products made from these fabrics are washed, manually or in a washing machine, using the “gentle mode” function at a temperature of 30-40 degrees, and after washing, the products are hung without wringing. You can iron these fabrics with a slightly warm iron. Exist
international designations of the conditions that must be observed during washing. A set of care symbols is printed on a special tape and sewn on from the wrong side.
To pin new theme we are working with an interactive test (Slide 20).
IV. Final part.
Evaluate student work in class.
Summing up, drawing conclusions (Slide 21)
Conclusion: in our life we need not only natural fabrics, but also fabrics made of chemical fibers. Who can imagine himself without an umbrella or a cool bag, and even a warm faux fur coat, on which there is no need to kill animals, is simply necessary for any self-respecting girl. And expensive natural fabrics are not affordable for everyone.
Therefore, the emergence of artificial tissues was due to economic benefits. The use of these fibers is their lower cost, which is explained by significantly lower labor costs for their production.
It's amazing how people used to do without such soft, durable and elastic artificial fabrics that bring warmth and comfort to our everyday life.
Informational resources:
- ABC of home economics G. Aslanov, E. Berezneva.
- Encyclopedia "History of Fashion".
- Magazines Burda, Moden, Diana, Glamour.
- http://www.gloryon.ru/ru/products/adv/200604_1/glossary_3.html (Types and properties of fabrics).
- Learn to sew R.I. Egorova, V.P. Monastery.
- Service labor D.N. Obraztsova, M.I. Ryzhechkin.
- Illustrated fashion encyclopedia by L. Kibalov, O. Gerbenov, M. Lamarov.
HISTORY OF THE APPEARANCE OF CHEMICAL FIBERS
The idea to artificially create fibers and threads resembling natural ones arose a long time ago. First, there was a desire to reproduce a twist, reminiscent of natural silk. It was valued very highly and was constantly in the center of attention of alchemists, just like gold and other precious metals. More than 300 years ago, in 1665, an outstanding English fThe scientist Robert Hooke, whose laws laid the foundation for the science of the strength of materials, published a treatise in which he logically approached the problem of obtaining artificial silk. Hooke wrote: “I often think that it is possible, apparently, to find ways to artificially obtain a sticky mass, similar to how it is formed in a silkworm, or even better. If such a mass can be found, then it seems to be an easier task to find a way to draw this mass into thin threads. I will not point out the benefits of such an invention, it is quite obvious ... "The scientist suggested that artificial fibers can be obtained from protein waste instead of natural silk.
Later, in 1734, the French naturalist Rene Antoine Réaumur tried to reproduce the process of isolating a silk thread by a silkworm caterpillar and obtaining a fiber, but similar in composition and properties to natural silk. He believed that silk was nothing more than a liquid resin that had been dried, and suggested that artificial silk could be obtained from various resins. But the low level of development of chemistry at that time did not allow scientists to solve the problem of creating artificial fibers.
Almost two centuries have passed since the publication of the logical calculations of Robert Hooke to the receipt of the first thread by chemical means. At first, only natural raw materials were used for the production of artificial fibers. And only in the 80s of the nineteenth century. botanist K. Negeli found that cotton consists of cellulose - that is, of the same substance as paper, which is obtained from wood.
The next was a patent issued in 1857 to Edward Joseph Hughes of Manchester. He proposed to obtain fiber from a mixture of fat, glue, flour, oil, gelatin and cellulose. However, it was not possible to implement his idea in practice.
Textile magazines of the time published a number of warning articles about the consequences of using man-made fiber clothing. The French government was forced to close the company, but Chardonnay continued to work on improving its production technology. And already in 1889, at the World Exhibition in Paris, the scientist presentednew samples of yarn and fabrics of industrial production.
His method was used to produce artificial silk in Germany, Switzerland and Belgium. But not only Chardonnay managed to get a synthetic fiber. In 1883, in England, D. Swan also obtained nitrocellulose, dissolved it in acetic acid, and, after passing through the finest holes, received threads. The inventor's daughters even made several tablecloths from the resulting fibers. These tablecloths were presented at an exhibition of inventions in 1885 as "artificial silk".
At the end of the nineteenth century. chemists have proposed another way to prepare "silk syrup" from cellulose. Cellulose was dissolved in the Schweitzer's reagent formed by the interaction of copper hydroxide and ammonia. From this solution, the new kind artificial silk - copper-ammonia. As in the production of nitro silk, a copper-ammonia solution of cellulose was forced through the thinnest holes, first into water, and then into a solution of sulfuric acid. In this case, the solvent was cleaved off, and filaments consisting of cellulose were obtained.
By 1900, the production of viscose fiber was already 1000 tons. The prospect of producing this fiber was obvious. Huge raw material resources and their cheapness have opened up wide opportunities for the production of viscose fibers. One cubic meter of wood yields 200 kg of pulp and approximately 150 kg of fiber, from which up to 1500 m of fabric can be produced.
Acetate fiber is produced from cellulose. Natural fibers were treated with acetic acid and then dissolved in acetone. Acetate threads were obtained from the solution. This method was first proposed in 1869, but practical application began only in 1890. In 1908, the production of protein fibers from milk casein was organized.
 Fibers obtained as a result of chemical processingnatural polymers of plant and animal origin are called artificial. Creating fibers from ready-made natural polymers, scientists came to the conclusion that you can learn how to create something similar yourself. As a result of many years of research in the 30s of the twentieth century. methods were developed for the synthesis of fiber-forming polymers consisting of the elements carbon, hydrogen, oxygen, nitrogen, etc. In practice, polymers are synthesized from compounds such as benzene, phenol, ethylene, acetylene, ammonia, hydrocyanic acid, which are produced in large quantities at chemical plants by processing of natural raw materials of oil, coal, gas. Such fibers are called synthetic.
Fibers obtained as a result of chemical processingnatural polymers of plant and animal origin are called artificial. Creating fibers from ready-made natural polymers, scientists came to the conclusion that you can learn how to create something similar yourself. As a result of many years of research in the 30s of the twentieth century. methods were developed for the synthesis of fiber-forming polymers consisting of the elements carbon, hydrogen, oxygen, nitrogen, etc. In practice, polymers are synthesized from compounds such as benzene, phenol, ethylene, acetylene, ammonia, hydrocyanic acid, which are produced in large quantities at chemical plants by processing of natural raw materials of oil, coal, gas. Such fibers are called synthetic.
Since the release of PVC fiber in Germany in 1932, a new era has begun in the textile industry.
The world's first nylon plant was opened in 1939. in the United States in Seaford, Delaware. It is curious that kapron, a common fiber-forming polyamide, was obtained 40 years earlier than nylon. In 1899, the German researchers Gabriel and Maas obtained the polymer E-caprolactam, but nothing followed this discovery.
In 1938, nylon was rediscovered, and again in Germany, by Paul Szlak, and he named it perlon. But the production of perlon during the Second World War was classified: the material was used for military needs - mainly for the production of parachutes and tire cord.
In Russia, nylon was first obtained in 1947, and since 1950, the production of lavsan began. It refers to polyester fibers and got its name in the laboratory of macromolecular compounds of the Academy of Sciences of the USSR. Polyacrylonitrile fibers (PAI) have been produced in the world since 1942, and in our country - since 1963. The world of chemical fibers is diverse. The production of these fibers is developing intensively, and at a faster pace than the production of artificial fibers. This is due to the availability of raw materials, less labor intensity production processes, a variety of properties of fabrics made from such fibers and their good quality.
Viscose fiber is pure cellulose obtained from spruce wood (chips) without any impurities. Depending on the purpose, viscose can have a shiny or matte surface. By changing the sheen, thickness and crimp of the fibers, viscose fabric can be given the appearance of silk, cotton or wool. Using thickened viscose threads, you can achieve an imitation of linen.
Viscose fabrics are inferior in strength to natural silk, although heavy-duty viscose fabrics are also produced. In the wet state, their strength is significantly reduced - by 50-60%. Viscose absorbs moisture better than cotton, but is inferior to it in wear resistance.
Viscose fibers burn in the same way as linen and cotton: quickly, evenly, with a bright flame, they smell like burnt paper, leaving easily crumbling light gray ash. Viscose fibers, unlike plant fibers, are sensitive to the action of alkalis and acids.
For acetate fiber, the raw material is wood and cotton waste. Acetate fiber silk fabrics look very similar to natural silk, have a shiny surface.
Acetate fiber fabrics do not absorb moisture well, but dry quickly; they have less strength than viscose, but greater elasticity, so they almost do not crumple, they retain their shape well. Acetate does not tolerate strong heat and melts at a temperature of 210 ° C.
Synthetic fiber fabrics
Synthetic fabrics are made from fibers obtained as a result of complex chemical reactions. They are different from each other chemical composition, properties, nature of combustion.
IN different countries these fibers are called differently, so we will focus only on the most common fibers and fabrics from them.
Fabrics made of polyester, lavsan, crimplene are soft and flexible, but very durable. They practically do not wrinkle, fix their shape well when heated, hold folds and pleats, do not fade in the sun, are not affected by moths and microorganisms. Their disadvantage is low hygroscopicity.
Nylon, capron, dederon are the most durable of all synthetic fibers. Fabrics made of these fibers are harsh to the touch, have a smooth surface, tear-resistant, abrasion-resistant, do not fade and wrinkle a little, are not affected by moths and microorganisms. Among the shortcomings can be noted poor absorbency and sensitivity to high temperatures.
Acrylic, nitron have the appearance of voluminous crimped fibers, so the fabrics from them are very similar to wool. They have the same properties as polyester fabrics, they are very sensitive to high temperatures: they quickly melt, acquiring Brown color, then burn with a smoky flame.
Elastane (lycra) is most often used in a mixture with other fibers. Elastane fibers are very elastic when stretched, able to increase their length seven times and then shrink back to their original size.
Fabrics with elastane are used in the manufacture of tight-fitting clothing: trousers, jeans, knitwear, hosiery. Such clothes are close to the figure and do not restrict movement. Products with elastane stretch well, wrinkle little and are durable.
Comparative characteristics The properties of fabrics made from various fibers are presented in Table 5. The fabrics are listed in descending order of properties.
Table 5. Comparative characteristics of the properties of fabrics from various fibers
|
Strength |
Shrinkage |
Hygro- |
elastic |
Washed- |
|
1. Elastane |
1. Elastane |
|||
|
2. Polyester |
2. Polyester |
|||
|
4. Viscose |
||||
|
5. Polyester |
||||
|
7. Viscose |
7. Viscose |
|||
|
9. Polyester |
9. Viscose |
|||
|
10. Elastane |
10. Elastane |
10. Wool |
Practical work No. 9
Determination of the composition of tissues and the study of their properties
Tools and materials: work box, samples of materials from cotton, linen, wool, natural silk, silk from artificial and synthetic fibers; saucer or cuvette with water; crucible for ignition of threads.
- Study the material in this paragraph.
- Choose six samples from all the materials offered.
- Determine by touch the degree of smoothness and softness of each sample.
- Determine the crease of the samples: hold each of them in your fist, hold for 30 seconds, and then open your palm.
- Remove two threads from each sample and soak one of them in a saucer of water. Break the dry thread first, then the wet thread. Determine if this changes their strength.
- Remove the thread from each sample and ignite in the crucible. Analyze the type of flame, the smell and the ash left after burning.
- Fill in workbook Table 6, noting the presence of a particular property.
Table 6. Determination of the composition of tissues by their properties
- Summarizing the obtained data, determine the raw composition of each tissue sample.
- Think about the composition of the fabric for the following products:
- summer dress;
- curtains;
- upholstery for furniture;
- nightshirt;
- sweater for winter views sports;
- swimsuit;
- umbrella;
- cloak.
New concepts
Viscose fiber, acetate fiber, synthetic fiber fabrics.
test questions
1. Why you need to know fibrous composition fabrics? 2. Where are chemical fiber fabrics used? 3. What properties do viscose fabrics have? 4. Clothes from what fabrics prevail in your wardrobe?
- Step by step recipes with photos
- Dish of legs and potatoes
- Pork Wiener Schnitzel
- Chicken Oatmeal Soup for Kids Oatmeal Chicken Soup
- What does it take to damage
- What is in cucumber
- Red (white) currant jelly
- The structure of the neon atom. Neon - what is it? Where is neon used Obtaining neon
- Electronic configuration of an atom
- Organic chemistry What science studies organic chemistry
- What is the difference between a teacher and a teacher - definition, features of professional activity Teacher and teacher what is the difference
- Markers of disorders of the nervous system What can affect the result
- Chirality and optical activity
- “A revolution is possible in the energy sector”: what scared the “generals” the re-elected Rafinat Yarullin
- Strong and weak electrolytes
- Chemical potentials of ideal gases
- Silk-screen printing wallpaper in the interior Flower wallpaper for walls silk-screen printing
- Chemical theory of solutions D
- Difference between adsorption and gel permeation chromatography
- Chemical theory of solutions Basic provisions of Mendeleev's theory of dissolution









